Bubble Membrane Mini-Lab, 2018
Simulation of a Membrane and Show Membrane Characteristics
Introduction
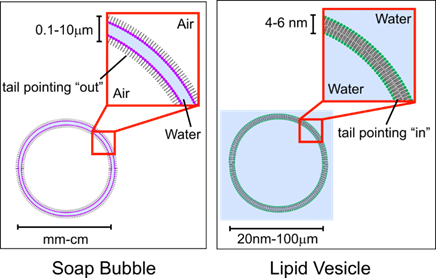
Bubbles make a great stand in for cell membranes. They’re fluid, flexible, and can self-repair. Bubbles and cell membranes are alike because their parts are so similar. If you could zoom down on a cell membrane, you’d see that much of the membrane is a double layer of little molecules called phospholipids. Phospholipids have a love-hate relationship with water. One end, the “head,” is attracted to water, and the other end, the “tail,” is repelled by water. Place phospholipids in water and they quickly form a double layer with the heads facing out on both sides. A soap molecule has the same split personality. However, with soap membranes, the hydrophobic tails of the phospholipids face “outward” and the heads face “inward”; opposite for what a cell membrane bilayer is in a cell or organelle membrane (see Figure).
|
Materials:
Groups of 3-5
1000mL Flask of Bubble Solution x # of Grps: 800mL Water 100mL Dish Soap 100mL Corn Syrup Stirring Rod/Stick for Solution Large Tray (4) Bendy Straws Blunt Probe
|
Stock Table
Thread Ruler(s) Scissors |
Steps:
1. Gather all material as directed; Note: get the thread and use the ruler / scissors later in the lab.
2. Stir your solution of 2 minutes then place the entire bottle into the tray.
3. Return the 1000mL flask and stirring rod/stick to the stock table.
Membrane Bubble Frame
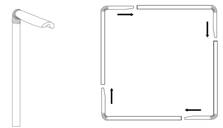
1. Bend 4 straws at elbows.
2. Flatten the shorter ends of straws and fold flatted surface in the middle.
3. Connect straws together by inserting short ends into long ends to create a strong square.
After performing each “Characteristics” (1-3), ask to be checked off:
Characteristic #1 “Flexing of a Membrane”
1. Lift frame out of solution at an angle while holding onto the two sides.
2. Tilt the frame back and forth and observe the surface of the film.
3. Notice the swirls of color as the light reflects off the film. Molecules in the cell membrane move about in the same way.
4. Hold the frame by the edges and rotate the sides in opposite directions. Notice the elasticity of the film just like any membrane.
5. Hold the bubble film parallel to the floor and gently move the frame up and down until the surface begins to bounce up and down.


Characteristic #2 “Merging Membranes”
1. Lift frame out of solution at an angle so that a thin film spans across frame.
2. Cover the surface of your finger or hand in bubble solution.
3. Slowly push your hand into and out of the film. The film should allow the hand to pass without breaking because they are covered with the “same” membranous solution. This is why structures with similar membranes can merge.


Characteristic #3 “Creating Pores and Channel Protein in the Membrane”
1. Obtain about 20 cm of heavy thread using scissors. Tie a loop around two fingers loosely and cut off extra thread.
2. Lift frame out of solution at an angle so that a thin film spans across frame. Then have someone hold the frame horizontally.
3. Making sure that the “thread loop” and fingers are very soapy, slip the loop off the fingers and gently down onto the film surface.
4. Use a blunt probe that has no soap on it and is completely dry to break the bubble film inside the loop of thread.
5. Insert a finger into middle of thread loop or move the frame around to simulate pore / integral protein movement.


