Preparation of Soap “Saponification” and the Study of Biological Fats 2014
Introduction

How is soap made and used? The purpose of this lab is to make a small batch of soap, test the soap's properties, and related the soap to biological characteristics. Triglycerides may undergo hydrolysis and/or bond breaking via heat (i.e. boiling) both in basic and acidic solutions to produce fatty acids and glycerol. Hydrolysis of "triacylglyceride" in a base solution (making soap) is called saponification. The Na+ salts of these, long-chain fatty acids are soaps. The best soaps are those in which the fatty acid salt is saturated. Potassium (K+) can be used as a Na+ substitute in the soap making process. Soaps and detergents are similar in that they both contain a long hydrocarbon chain and a highly ionic or polar end. Calcium chloride (CaO2) in hard water may hinder the ability of soap in that the Ca++ may replace the Na+ and make the molecule less attracted to water (ie. Ca(C18H35O2)) and less effective as a cleaning agent; Ma++ has a similar effect. Soap's general characteristic is that it emulsifies or separates oily material from a watery environment and is basic due to the -OH or saponification process. The ionic ends of soap are water soluble and thus attracted to water; while the hydrocarbon chain is not soluble in water and thus attracted to oil/dirt. When water is used to rinse, the hydrophilic ionic end "drags" the oil/dirt with the water because it is also attached to the hydrophobic hydrocarbon chain that is attracted to the oil/dirt. Indicators such as phenolphthalein will turn a basic solution red-pink. The amphiphilic properties of soap can be found in the human body in the form of bile salts from the liver and phospholipids in cellular membranes. The hypothesis is that if soap can be made, then tests for "soap-like" characteristics can be performed to confirm the soap’s lipid-like qualities.
General Soap: Using Na+ (may use K+)
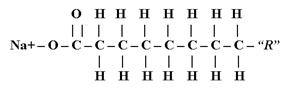

Method
Apparatii (per group)
10-12 mL of Crisco (~20g of Crisco)
15 mL of 6 M NaOH (ie 24 g NaOH in 100 mL of water)) ; (KOH can be used as a substitute)
50 mL of 30% NaCl (use “only” Canning Salt" to create solution: 30g/100mL of distilled water)
50 mL of distilled water
5 mL of vegetable oil
Hard Water from tap (or 0.1 M CaCl2 solution)
Phenolphthalein solution
Sudan III or IV
Commercially Prepared Dry Soap (flakes)
(2) 250 ml beaker
(9) test tubes
25 mL graduated cylinder
test tube rack
stirring glass (not plastic) rod
funnel
sharp edge (ie scalpel/knife)
eye dropper
filter paper
1 pair of gloves
goggles
heat source
scale (with disposable weighing dishes)
strip of masking tape
fine tip marker
paper towel(s)
references

Procedure A: Making the Soap
1. Weigh about 20 g of Crisco using an electronic scale.
2. Place the Crisco in a 250 mL beaker and heat slowly in order to melt the fat. When fat is melted remove from burner for about 5 minutes.
3. Wearing gloves and goggles. Add about 15 mL 6M sodium (or potassium, whatever will be available) hydroxide solution: Caution: The solution will irritate the skin.
4. Return the beaker with the fat and hydroxide solution to the heat source and boil the mixture while stirring with a glass stirring rod. Keep gloves and goggles on.
5. Continue stirring for ~15 minutes or until the liquid appears to be gone; place heater on a lower setting towards the end of the boiling process. It is important to remain stirring to prevent "spattering". “Foaming” is alright, just don’t let it foam too many times.
6. Remove beaker from the heat and allow to cool. The soap is ready if it appears as a white granular semi-liquid material. Wait for about 3-5 minutes to cool.
7. Add 25 mL of 30% sodium chloride solution and break up any lumps with the glass rod. This will help remove remaining glycerol and excess sodium hydroxide (or potassium hydroxide); plus, insure that the fatty acids have Na+’s (K+’s).
8. Place a funnel lined with filter paper onto the other 250 mL beaker.
9. Decant "filter" the soap solution by carefully pouring it into the lined funnel and allowing the liquid to pass through.
10. Wash the soap one more time with 25 mL of 30% sodium chloride solution by pouring the solution onto the soap that is still in the filter lined funnel. Again, to remove remaining glycerol and excess sodium hydroxide (potassium hydroxide); plus, insure that the fatty acids have Na+’s (K+’s).

Tests were then done to acquire pH levels, emulsification and hard water properties:
